How Does Sugar Affect the Viscosity of Water
In conclusion the sugar had an effect on the waters viscosity because it thickened as the water was near room temperature and thinned as it boiled and or was colder than room temperature. For this experiment I took two tall glasses and filled them both with water up to the same amount.

Full Article Viscosity Of Aqueous Carbohydrate Solutions At Different Temperatures And Concentrations
How does sugar affect the viscosity of water.
. Viscosity could be reduced by increasing the ionic strength or temperature. How to calculate the viscosity of water. Thing to add to water to increase viscosity is sugar.
Does sugar increase the viscosity of water. The viscosities of liquidsolid suspensions were experimentally determined for castor oil-paraffin and acrylonitrile butadiene styrene ABS plastic particle systems at room temperature and for a Fe C melt at various temperatures. I believe the more sugar that is added to the water will increase the viscosity of the water due to the fact that certain quantities of sugar are incorporated and broken down into the water.
How does sugar affect the viscosity of water. Only data on single sugar water solutions are available in the. The more sugar is added the more viscous the water gets and the thicker it becomes.
Does sugar affect the viscosity of water. Does salt affect the viscosity of water. Viscous force resist the.
Soaps and detergents have chains of hydrocarbons attached to water soluble groups. Adding heat to the mixture will help in mixing more sugar into the water. Risks - dropping or breaking glass beaker - spilling and slipping on water Precautions - handle all items with care - maintain a dry surface 1.
Answer 1 of 4. Adding substances that make water thick like sugar increases the viscosity of water. There are a few ways to increase the viscosity of water.
However when comparing the solutions in terms of grams of solute. The effect by sugars was enhanced at higher protein concentrations. In this calculator you will learn what the absolute viscosity of.
When comparing the solutions in terms of solute concentration the sugar solutions caused a greater increase in the viscosity of water. People can use this everyday because this can come in handy for the consistency of what syrup you want. Viscosity of the sugar solution by the marbles velocity it is necessary to find the finite value of the variables.
In one of the glasses I added two teaspoons of sugar and in the other one I just left it as it was. This water viscosity calculator will help you determine the viscosity of water at room temperature or at any temperature even those above 300 C. This allows the water to spread more easily over a surface and to reduce or eliminate the tendency for the water to bead up on a surface.
Water has a viscosity a resistance to shear due to the fact that water molecules have some attraction for each other due to the polarity of the molecules The protons bound to their own oxygen still have some attraction to other oxygens. Because perspectives can differ neither solute consistently affects the viscosity of water more than the other. Concentration and temperature effect on the viscosity of binary sugar solutions.
What soap or detergents etc do is to reduce the surface tension of water. The viscocity is 94 How does. These two are the forces which act perpendicular to each other viscosity is horizontal and buoyancy is in vertical.
But the viscosity of the water itself is unaffected. Answer 1 of 2. Thus the radius and the mass of the marble were measured in order to calculate the density of the ball.
The degree of sphericity of the particles was introduced as a parameter for characterizing the effect of the particle shape on the apparent viscosity of liquid. First of all let me tell you the basic difference between the viscosity and buoyancy. A possible alternative hypothesis is that the perception of viscosity itself affects overall flavour perception.
The water itself is Newtonian but the introduction of the polymer in concentrations by weight as low as 02 can have a large affect on its rheological behavior. A change in viscosity based on the speed or force used it may increase or decrease. This difference in water solubility of the different parts of the detergent will cause it to align in various geometrical patterns.
If I add more sugar to the water then the viscosity of the sugar water will increase because the sugar will cause the substance to become thicker. In fact soap does not affect the viscosity of water in any way. The addition of sugars produced a linear increase of the specific viscosity at decreasing water activities.
The rates of increase slopes were proportional to the hydration capacity of each sugar. When comparing the solutions in terms of solute concentration the sugar solutions caused a greater increase in the viscosity of water. Temperature also changes the viscosity of honey and heat is.
The effect of sugar solution type sugar. The viscosity of honey depends on the amount of water and the type and amount of sugar contained in it. Similar effects of sugars were observed in the two mAbs examined.
The effect may be explained by commonly accepted mechanisms of interactions between sugar and protein molecules in solution. Dinh Huu Nhan HYPOTHESIS Conclusion When an amount of salt is dissolved to the water the water will be more viscous. Therefore a dishwashing liquid or reagent does not affect the viscosity of water it only affect its surface tension.
Furthermore dissociation of free water molecules arranged around the periphery of the sugar molecule produces a high membrane potential across the taste cell thereby enhancing sweetness perception. Increasing the water concentration will reduce the viscosity of the honey. Get started for FREE Continue.
For each trial certain cups of sugar was put in a pot with a boiled water to dissolve the sugar. Gases with little attraction to othe. What is the Viscosity of 60 sugar and 40 water solution.
How does salt affect the viscosity of water. However when comparing the solutions in terms of grams of solute added the salt solutions caused a greater increase in the viscosity of water. Soaps and sythetic detergents affect the viscosity of water in many different ways.
This can be proven because as the the sea water is viscous than than normal water so salt will make the water more.

Viscosity Of Sucrose Solution In Various Concentrations At 303 K Download Scientific Diagram

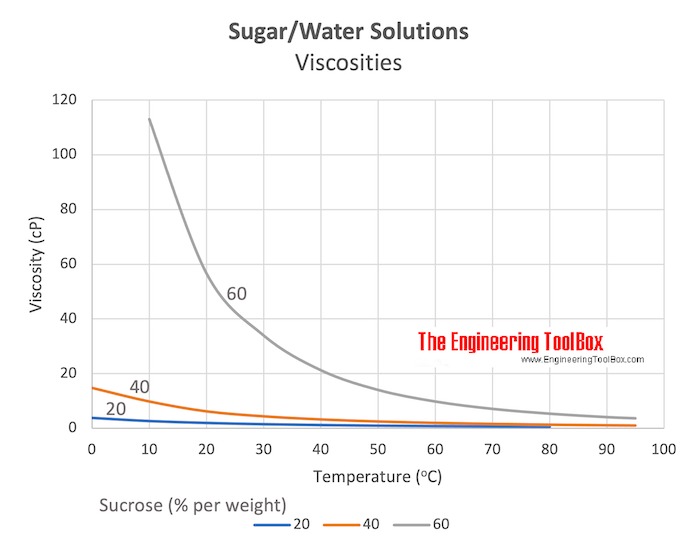
Sugar Water Solutions Viscosities

Viscosity Of Sucrose Solution In Various Concentrations At 303 K Download Scientific Diagram
0 Response to "How Does Sugar Affect the Viscosity of Water"
Post a Comment